
How many neutrons in a krypton atom 48 You will need to obtain a periodic table. How many neutrons does Krypton-86 isotope have Answer: 50. The element krypton has an atomic number of 36 and a mass number of 84. It has no electric charge and a rest mass equal to 1.67492749804 × 10 − 27 kg-marginally greater than that of the proton but 1,838.68 times greater than that of the electron. What is isotopic mass for Krypton-86 isotope Answer: 85.910611. Neutron, neutral subatomic particle that is a constituent of every atomic nucleus except ordinary hydrogen.

The removal or addition of electrons to a neutral atom creates ions that have a net negative or positive charge. The number of electrons on a neutral atom is equal to the number of protons in the nucleus of the atom. If the mass number of a krypton atom is 84, which answer shows the number of subatomic particles inside and outside the. The particle derives its name from the fact that it has no electrical charge it is neutral. Are neutrons in every atom?Ī neutron is a subatomic particle found in the nucleus of every atom except that of simple hydrogen. An isolated neutron is unstable and will decay with a half-life of 10.5 minutes. However within a nucleus, the beta decay process can change a proton to a neutron.
KRYPTON NUMBER OF NEUTRONS PLUS
) which is slightly radioactive with an extremely long half-life, plus traces of radioisotopes that are produced by cosmic rays in the atmosphere. 4 5 Naturally occurring krypton is made of five stable isotopes and one ( 78. To the best of our knowledge, an isolated proton, a hydrogen nucleus with or without an electron, does not decay. There are 34 known isotopes of krypton ( 36 Kr) with atomic mass numbers from 69 through 102. Hydrogen, Helium, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Phosphorous, Sulphur, Chlorine, and Argon are the non-metals in the first twenty elements. Lithium, Beryllium, Sodium, Magnesium, Aluminium, Potassium, and Calcium are metals in the first twenty elements. Therefore, a krypton atom has forty-eight neutrons. It was discovered in 1898 by William Ramsay and his assistant Morris Travers. The difference between the mass number of the krypton atom and the number of protons is forty-eight. The chemical element krypton is classed as a noble gas and a nonmetal. Krypton discharges a greenish yellow glow when it is mixed with other gases. There are 34 known isotopes of krypton ( 36 Kr) with atomic mass numbers from 69 through 102. Krypton behaves as Neon at very high temperatures but glows purple instead of red. Krypton has 36 protons and 48 neutrons in its nucleus giving it an Atomic Number of 36 and an atomic mass of 84. Unusual Facts about Krypton? Krypton was discovered partially by accident, that is why it is named after a Greek word “Krypto”, means “hidden”. What are 3 interesting facts about krypton? Use the Periodic Table of Elements to complete the following chart. Give the symbol and number of electrons in a neutral atom of: Uranium. It has a melting point of -157°c and a boiling point of -153°c.It’s not green and it doesn’t glow, but a mineral discovered in a Serbian mine has the same chemical composition as Kryptonite, the cartoon-conceived bane of Superman. The number of protons in one atom of an element determines the atoms. It is located in Group 18 as a non metal gas which is odourless and colourless. Krypton’s main uses are in photography equipment for lighting and in high powered lasers. They named the new gas Krypton, from the Greek ‘kryptos’ meaning ‘hidden.’ Krypton as a gas was used as a scientific length between 19 as the measurement of a metre being defined as 1 650 763.73 wavelengths of krypton-86’s orange-red spectral line. After removing Nitrogen and Oxygen by heating with Copper and Magnesium, they applied a high voltage to the left over gases and produced a yellow and green spectrum, which had never been seen before. Obtaining a sample of air as a liquid they began to evaporate off gases. He had built up a picture of the gases existing in the same group. Ramsey had a track record of discovering Noble gases having discovered helium and argon.
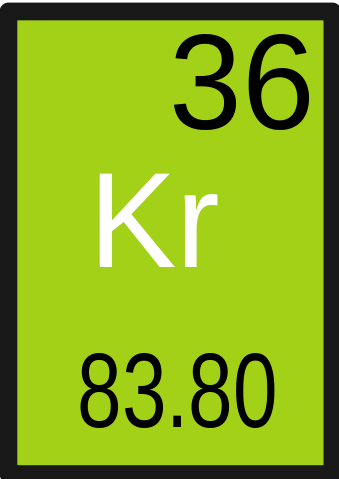
It was discovered in 1898 by Scottish chemist William Ramsey and his assistant Morris Travers. Krypton like most Noble gases is odourless, colourless and inert. It has the atomic number 36 in the periodic table and belongs in Group 18, the Noble Gases. INTERACTIVE EXAMPLE Calculating Mass Number Determine the symbol for the krypton atom (Z 36) that has 48 neutrons.

Krypton (Kr) exists as a colourless, odourless gas and is chemically inert.


 0 kommentar(er)
0 kommentar(er)
